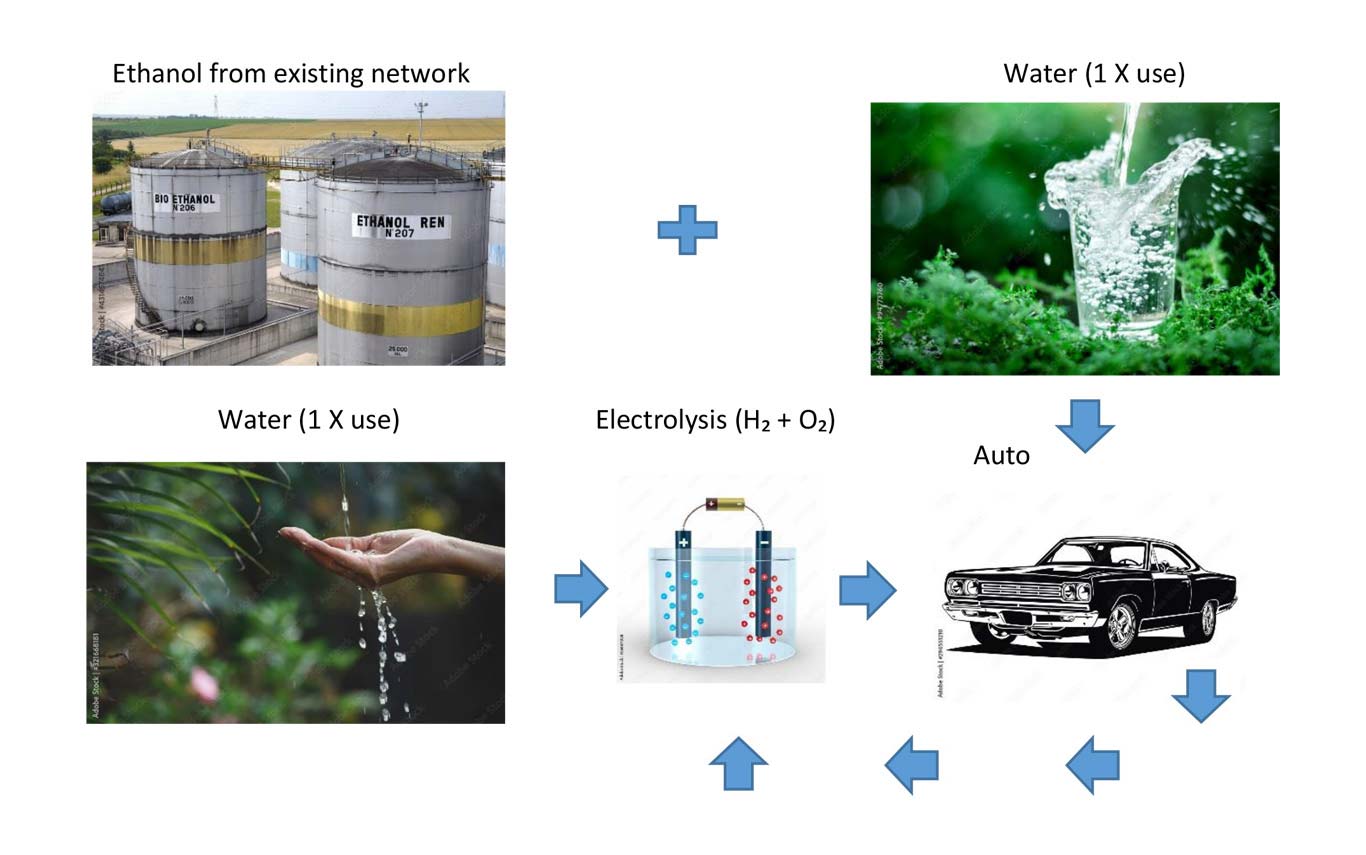
We produce a water-vapor (an additive) which is injected into the combustion chamber of both gasoline and diesel motors.
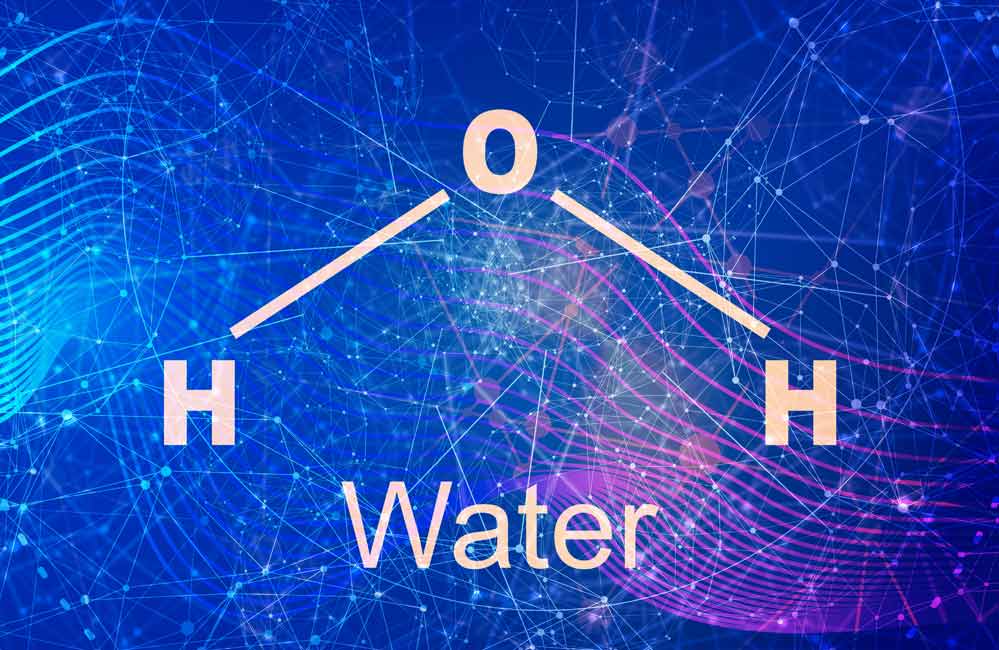
The basic technology is called electrolysis which goes back about 170 years to the 19th century scientists Nicholson, Carlisle and Faraday who were first able to split the water molecule (H2O) with the help of direct (battery) current and two metal plates submerged in water. The picture on the right shows the water molecule with the larger Oxygen (O) atom in the middle and the two smaller Hydrogen (H) atoms in their normal positions about 104° apart.
According to theory, when the water molecule is pulled apart by the direct current, the 104° angle no longer exists and the water turns into the gases O and H. The negative charged Oxygen atoms (now a gas) go to the positive plate (anode) and the positive Hydrogen atoms (also gas) go to the negative plate (cathode). The two gases (H and O) then rise to the surface and can be captured for use in a variety of applications.
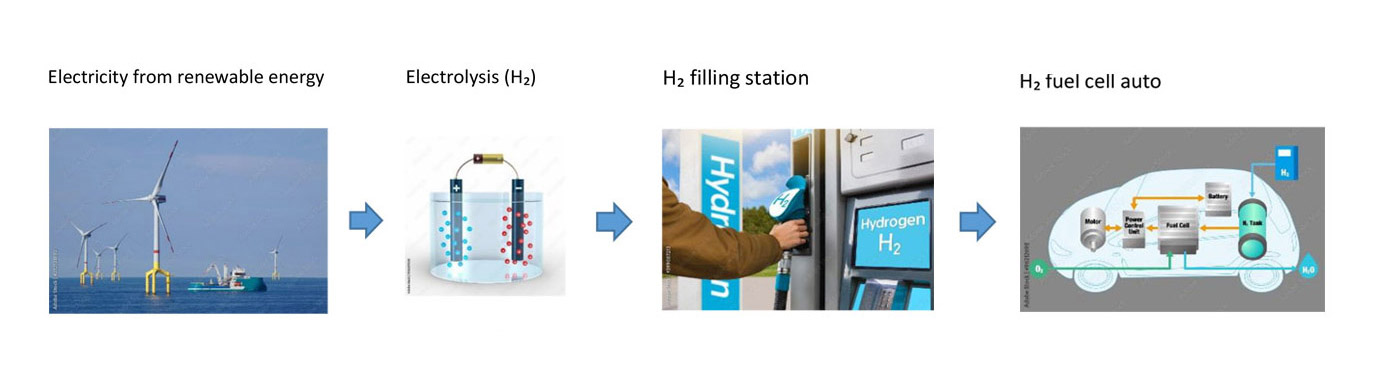
This basic process, splitting the water molecule, is still used today on an industrial scale to make “green” hydrogen for use in fuel cells. Green hydrogen is produced when renewable electricity from wind generators, solar panels or water power is used in the electrolysis process. Gray hydrogen is produced from natural gas.